Abstract
With the emergence of targeted therapies, defining the best strategy for the treatment of previously untreated CLL patients remains challenging. The aim of this phase 2 study was to compare the efficacy in first line of combined ibrutinib and venetoclax (IV) to the standard FCR regimen in fit patients with CLL of intermediate risk defined by either unmutated IGHV status, 11q deletion or complex karyotype in the absence of TP53 alteration.
As previously reported (Michallet et al., Blood 2021; 138 (suppl 1):641), 120 patients were randomized 1:1 between two treatment arms, ie FCR 6 cycles or IV. After a lead-in phase of ibrutinib as a single agent from month (M)1 to M3, the total duration of treatment with the IV combined treatment was based on the response achieved at M9; if bone marrow (BM) MRD was < 0.01% using flow cytometry, the treatment was continued for 6 additional months until M15 and then stopped; if BM MRD at M9 was ≥ 0.01%, the treatment with IV was continued for 18 additional months until M27. PB MRD evaluation was performed every 6 months, whereas BM MRD was performed at M9 and M27.The primary endpoint was the percentage of patients with BM MRD < 0.01% at M27 in both arms. We present herein safety data and the preliminary results of the PB MRD evaluation at M15 .
These 120 patients were enrolled from September 2019 to February 2021. The median age was 59 [34-72] and 61 [34-74] years in the FCR and IV arms, respectively. The characteristics of patients were well balanced between the 2 arms in terms of gender (male 72% FCR, 74% IV), PS ECOG 0-1 (59% FCR, 68% IV) and Binet stage (A, B and C 15%, 64%, 21% for FCR ; 8.5%, 59% and 32% for IV). 11q deletion was found in 20% and 24% of cases in the FCR and IV arms, respectively and all patients but one had unmutated IGHV.
At the time of data cut-off for this interim analysis, the median follow-up was 24.8 [16.7 - 33.6] months. Sixty-three serious adverse events (SAE) were reported so far, 33 in the FCR arm and 30 in the IV arm. In the FCR arm, the most frequent SAE were tumour lysis syndrome (N=3), infections (N=12 including 4 COVID19 infections), febrile neutropenia (N=5) and secondary malignancies (N=2, including 1 myelodysplastic syndrome and 1 acute myeloid leukemia). In the IV arm, the most frequently reported SAE were tumour lysis syndrome (N=5), infections (N=8 including 4 COVID19 infections), cardiovascular events (N=7), acute renal failure (N=2) and secondary malignancies (N=2, including 1 colorectal cancer and 1 skin basal cell carcinoma). Three grade 5 adverse events were reported consisting in 1 acute myeloid leukemia in the FCR arm and 2 sudden death in the IV arm. No patient died because of COVID19 in the study.
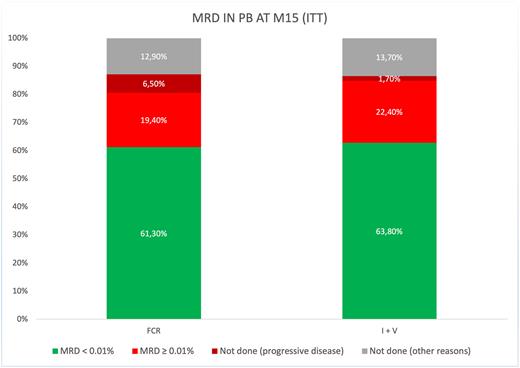
At M15, in intention to treat, in the FCR arm, 61.3 % of patients had peripherical blood MRD <0.01%, whereas MRD was ≥ 0.01% without clinical progression in 19.4% of patients and 6.5% of patients had experienced clinical progression. In the IV arm, PB MRD < 0.01% was reached in 63.8% of cases, MRD was ≥ 0.01% in 29.4% of patients and clinical progression was reported in 1.7% of patients (figure 1). In the FCR arm, 1 patient with MRD ≥ 0.01% at M9 converted to MRD < 0.01% at M15 while 2 patients had a signigicant increase of their PB MRD level from <0.01 to ≥ 0.01%. Conversely, in the IV arm, 12 patients converted from MRD ≥ 0.01% at M9 to MRD < 0.01% at M15 while 2 patients increased their PB MRD level from <0.01 to ≥ 0.01%. The two later patients had prematurely discontinued treatment before M15 evaluation.
In conclusion, these preliminary results show an increase in PB MRD < 0.01% rates between M9 and M15 in the IV arm, with rates similar to those of the FCR arm at M15. However, toxicity remains significant. The primary enpoint analysis at M27 will be of great interest to try to determine the best strategy.
Disclosures
Quinquenel:Janssen: Honoraria; Abbvie: Honoraria; Beigene: Honoraria; AstraZeneca: Honoraria. Letestu:Abbvie: Consultancy, Other: Funding for congress expenses, Speakers Bureau; AstraZeneca: Speakers Bureau. Le Garff-Tavernier:Abbvie: Consultancy, Honoraria; Janssen: Honoraria. Laribi:AbbVie, AstraZeneca, Beigene, Iqone, Janssen, Novartis, Takeda: Honoraria. Leblond:Abbvie, Beigene, Roche, Amgen, Lilly AstraZeneca, Janssen, Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees. Dartigeas:Janssen, Abbvie, Roche, AstraZeneca: Other: Travel Grant; Abbvie, Roche, Janssen, Beigene, AstraZeneca: Membership on an entity's Board of Directors or advisory committees. Guieze:Astrazeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Beigene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie, Beigene, Janssen, Gilead, Roche, AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees. Tournilhac:IdeoGen: Honoraria, Other: Travel grant , Research Funding; Abbvie: Honoraria, Other: Travel grant , Research Funding; Takeda: Honoraria, Other: Travel grant , Research Funding; Securabio: Honoraria, Other: Travel grant , Research Funding; Gilead: Honoraria, Other: Travel grant , Research Funding; Janssen: Honoraria, Other: Travel grant , Research Funding. de Guibert:Janssen: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria. Feugier:AstraZeneca, Janssen, Abbvie, Beigene, Gilead: Membership on an entity's Board of Directors or advisory committees, Other: Congress Invitations. Lepretre:AbbVie, Roche, Amgen, AstraZeneca, Janssen, Beigene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Dupuis:abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees. Cartron:MabQi, Ownards Therapeutics, Abbvie, Roche, Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Gilead, Novartis, Mylteni, Sanofi, Abbvie, Takeda, Roche, Janssen, Celgene, Novartis, Bristol Myers Squibb: Honoraria. Ysebaert:Abbvie, Astra-Zeneca, Janssen, Roche, Beigene, BMS/Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Delmer:AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support; Novartis: Honoraria; Takeda: Honoraria; Abbvie: Honoraria; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel Support.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal